Concise Initial Project Planning: Enable Successful Development of Medical Devices
With a plethora of standards and regulations, the development of a medical device places high demands on structure and documentation in parallel with product development.
Structured project planning and execution based on experience and best practices is a key success factor which is often underestimated.
Good initial overall planning by an experienced practitioner is a quick and inexpensive way to take out risk. In conjunction with appropriate training, controlling, project risk management and change management, it efficiently leads to a predictable result in which the risks of massive rework are contained.
The Challenge: Good Initial Planning
In an environment with a large number of confusing standards and regulations, the development of a medical device places high demands on structure and documentation in parallel with product development. Structured project planning and implementation based on experience and proven practices is a key success factor that is often underestimated.
Good initial overall planning by an experienced practitioner in project planning and project management in medical device development based on proven methodologies can be obtained quickly and inexpensively and, with appropriate training, controlling, project risk management and change management, leads to a predictable result in which the risks of massive rework are low.
![[Ziele -> Was -> Wie -> Wer -> Wann --> Projekterfolg]](https://www.ctk-medical.com/wp-content/uploads/2025/04/Projektplanung-Elemente_en.png)
Create Structures, Gain Experience
In established companies, even less experienced project managers can rely on proven structures, processes and experience of colleagues from successful development projects from which they can derive sensible and efficient structures and plans.
However, startups and companies that plan to develop according to MDR/FDA regulations for the first time face a mountain of challenges: They have to break down their overarching development goals into good sub- and intermediate goals, plan and develop the “what”, “who” and “how” (processes) in parallel and understand and keep an eye on all relevant aspects.
![[Von Idee und Prototyp - Die Herausforderungen aus Normen, Patientensicherheit, klinischem Nutzen, strukturierter Entwicklung, Kosten, QMS, Registrierung, Safety, Technischer Dokumentation und Business Case überwinden - um zum zugelassenen Produkt zu kommen]](https://www.ctk-medical.com/wp-content/uploads/2025/04/Medizintechnik-Herausforderungen-en.png)
Feasibility Proven – but there is Still a Long Way to Go
In most cases, initial prototypes are created without specification and risk assessment. This shows the feasibility with little effort, opens the way to a successful product and helps to convince management and/or investors.
My cooperation with customers often begins with the question: How do I get from here to a product and technical documentation that can be approved? I have experienced several times that this path is severely underestimated.
In order to efficiently achieve a safe and compliant product, the actual product development begins with intended use and stakeholder requirements. If not already established, efficient and standard-compliant processes must be described, introduced and trained in parallel. The necessary investment is usually significantly higher than what went into creating the prototype.
The necessary systematic way of working sets intermediate goals, controls progress and only moves on when the goals have been achieved. Changing work habits requires patience and perseverance. It is not for nothing that more than 70% of startups fail before their product is on the market or they are acquired by a larger company.
What makes medical device development complex?
The authorities, both FDA and in the EU, require “fully” documented descriptions of activities and results. This means:
- Documentation – Activities and results that describe, verify and validate the product must be documented and tested at least according to the 4-eyes principle.
- Documented planning – Activities must be planned before they are carried out. The planning must be documented, both the “what” and the “how” (processes).
- Qualification – Anyone who carries out an activity must be proven to be suitably qualified
With the main objectives:
- Clinical Benefit – The product meets the specified medical benefit as demonstrated by clinical validation
- Safety – The product is proven to be safe for patients and users, “all” risks to patients and users are considered and addressed through measures as far as possible; the product is fit for use and cyber-safe; the product does not exhibit unplanned behaviors that endanger patient/user safety
- Completeness – All functions of the product are described and the functionality is proven (verification)
These requirements derive from proven systems and software engineering practices: They ask for a structured approach from requirements analysis to architecture and design to test and validation, similar to other regulated areas or industries with risk potential, such as automotive, rail and aerospace.
A rapidly developed prototype will usually not meet these requirements, even through rework. A restart if development, perhaps reusing insulated modules, is usually the more efficient way:
- Product specification – clinical benefit, boundary conditions, intended use, product environment
- Identify product requirements – features, restrictions, normative, safety and security requirements
- Identify patient and user risks and define risk-mitigating measures
- Modular, expandable, robust system and software architecture
- Iterative / Agile development: detailed specification, modular implementation, test protocols
- Verification and validation as final confirmation
- In parallel Clinical Validation
Efficient and Concise Estimation, Planning and Realization – Even the First Time
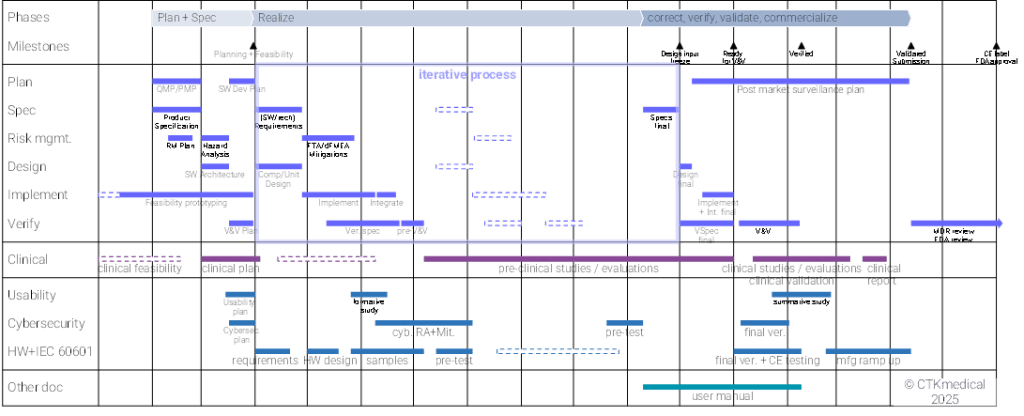
With experience, it requires comparatively low costs and effort to set up a meaningful and goal-oriented development plan that creates a basis for successful development through good specifications, risk analysis and architecture, and convinces management or investors.
Without experienced, trustworthy and reliable colleagues or development partners, it is virtually impossible to answer the underlying key questions, and to start a project with an understanding of the necessary details:
- What happens sensibly in the development of the planned medical device, why is it necessary / sensible, and in what order? How does it have to be documented so that it is audit-proof?
- How do I efficiently interleave the step-by-step development of a QMS and standards-compliant processes with basic specifications, product risk analysis, a suitable architecture, the actual development and later successful and complete verification and validation as well as production planning? How do I map “usability” and “cybersecurity”? How much time and effort do I need to plan in sum?
- How can I leverage iterative / Agile development to eliminate risks at an early stage and avoid a long rework time until the product is stable and verified?
- How do I manage such a project sensibly and efficiently, from meetings to work package planning and tracking to understanding and managing risks and changes, with reviews and approvals, so that in the end there is a safe product and approval-ready technical documentation with limited risk on time and cost?
Training and self-study cannot replace experience. Targeted experience and support, both in medical device development and in successful iterative project management, can help the team to gain its own experience during development and successfully master the challenges.
My Offer
- A free initial discussion of 1 to 2 hours will allow you to present your project and status. I will touch on the the key elements of success from my experience. Together, we will identify the most important gaps and topics of concern.
- Your IP is safe: Let’s sign a Non-Disclosure-Agreement before talking about your project: NDA
- Subsequently, at a fixed price, I offer to create a viable basic planning for you, based on a one-day workshop.
- Where desired, I will support you in your further project, in project planning and project management, in the development and training of standard-compliant methodologies and processes (QMS) as well as in quality assurance during development.
![[Bild Christoph Taegert-Kilger]](https://www.ctk-medical.com/wp-content/uploads/2025/02/portrait_ctk_600px-1.png)
Christoph Taegert-Kilger
As an experienced project manager, system/software architect and quality manager, I support startups and established companies with best practices of system and software engineering, from idea and prototype to approved medical device.